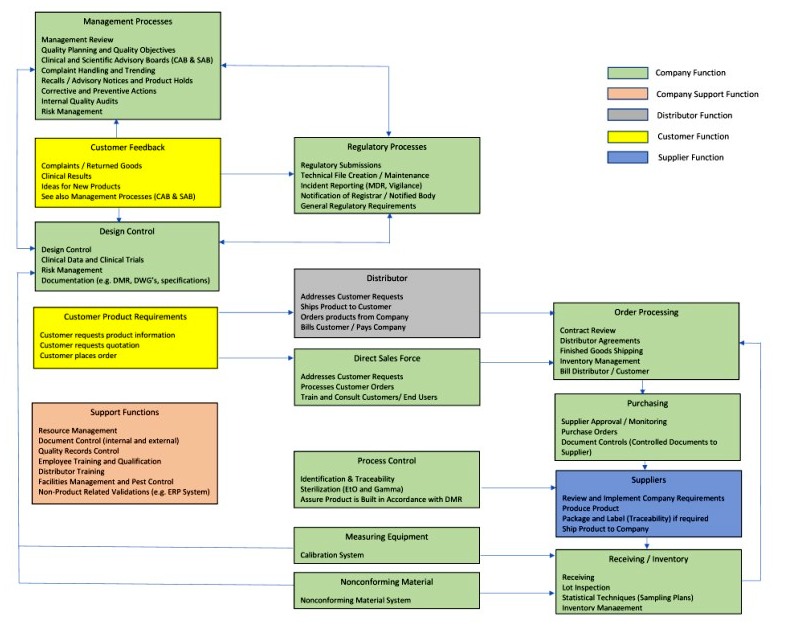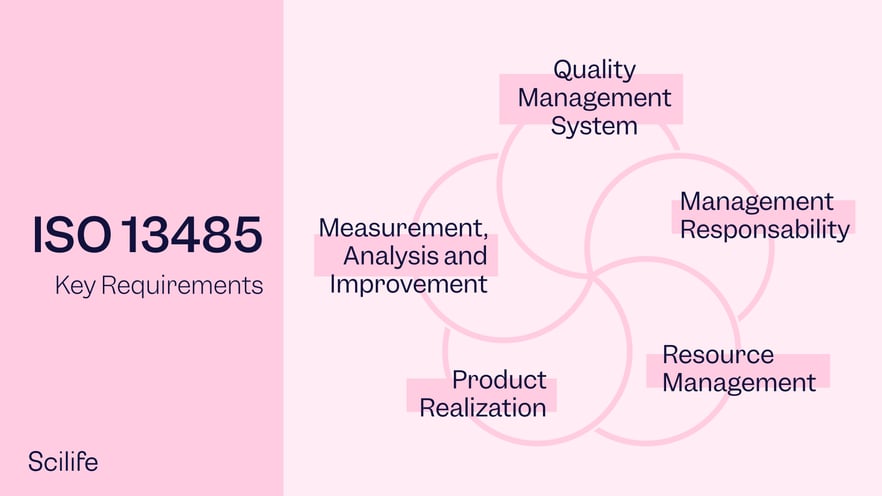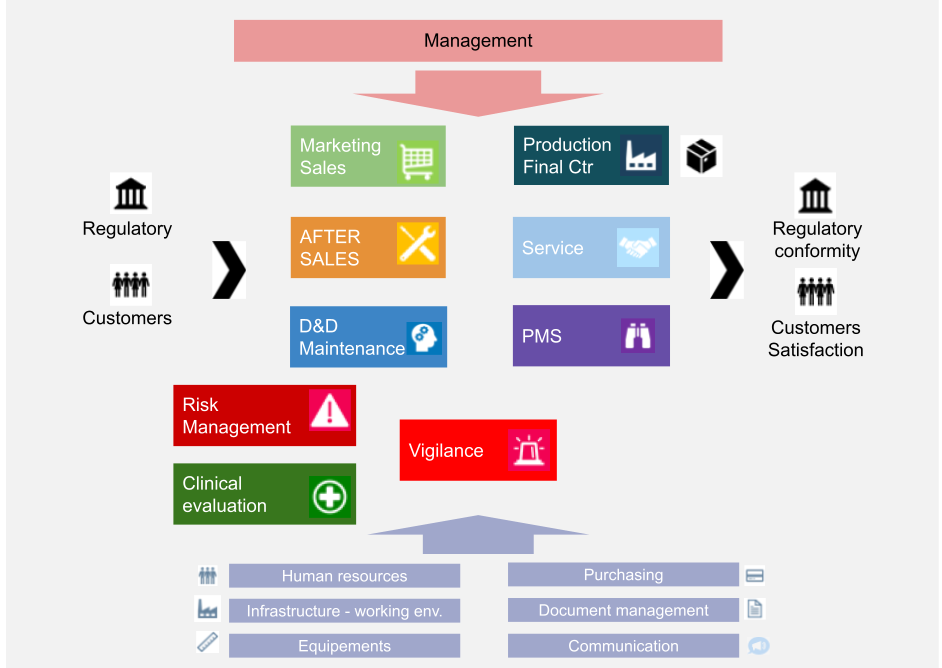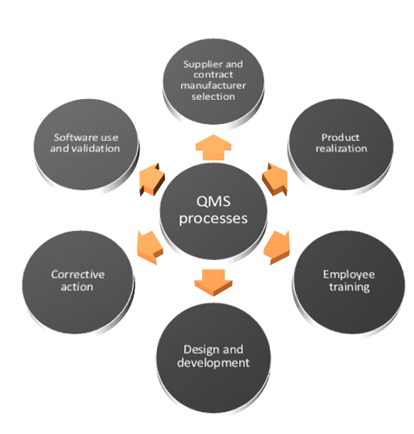
Importance of Quality Management System in Medical Device Manufacturing | Medical Device Johari Digital Healthcare Ltd.
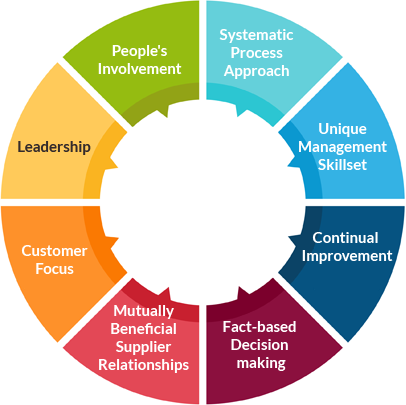
ISO 13485:2016 Medical Devices Quality Management Systems Certification in India, Chennai | Traibcert.in
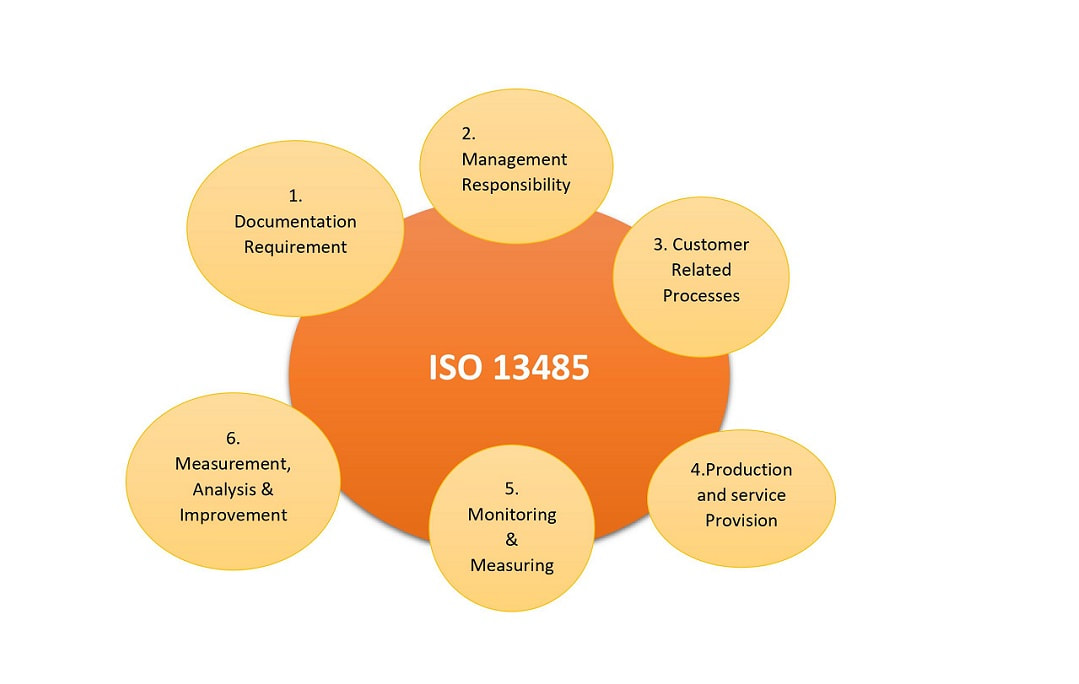
ISO 13485 Quality Management System for Medical Devices Certification Consulting - GlenView Group, Inc. - ISO Lead Auditor Certification Courses - Consulting & Training

QA International Certification Ltd - Medical Devices- Quality Management System (ISO 13485:2016) Safety and quality are non-negotiable in the medical devices industry, that is why we Have standard ISO 13485. Regulatory requirements

ISO 13485 – Medical Device Quality Management System Requirements – ISO Templates and Documents Download